Although titanium dioxide photocatalysts having anatase phase are a promising substrate for photodegradation of pollutants in water and air, their photocatalytic activities show only under UV light. For us to utilized a wide range of incident light such as solar light, development of the photocatalysts whose activities show under visible light is one of the most important strategies. We have synthesized chemically modified titanium dioxide photocatalysts in which S (S4+) substitutes for some of the lattice titanium atoms. They show strong absorption for visible light and high activities for degradation of methylene blue, 2-propanol in aqueous solution and partial oxidation of adamantane under irradiation at wavelengths longer than 440 nm. The oxidation state of the S atoms incorporated into the TiO2 particles is determined to be mainly S4+ from XPS spectra.

Figure 1. Photograph of TiO2 Photocatalysts
Nanoscale Surface Structure Controle of TiO2 Photocatalysts
A titanium dioxide powder consisting of 1-mm-size rutile and anatase particles was obtained, on which developed crystal faces were observed by a scanning electron microscope. From electron diffraction analyses, it was found that the rutile particles exposed {011} and {110} crystal faces, and the anatase particles exposed {001} and {011} faces. This powder showed high activity for some photocatalytic reactions, including oxidation of water. After photocatalytic oxidation of water on the powder using hexachloroplatinate(IV) ions as the electron acceptors, Pt deposits were observed mostly on the rutile particles, especially on the {110} face. When 2-propanol was added to the solution, Pt was deposited on both the anatase and rutile particles. Using thus prepared Pt-deposited TiO2 powder, Pb2+ ions were photocatalytically oxidized into PbO2. After this reaction, PbO2 deposits were seen on the {011} face of the rutile particles. On the anatase particles, PbO2 deposits were observed in a larger amount on the {001} face than on the {011} face. These results indicate that the crystal faces help the separation of electrons and holes, and that this effect is stronger for the rutile particles than for the anatase particles.

Anatase TiO2 Rutile
TiO2
Surface Structure Controled TiO2 Photocatalysts
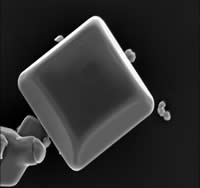
In addition to the crystal structure of TiO2 powders for improving a photocatalytic activity, the property of surface of TiO2 photocatalysts is one of an important factor to determine their photocatalytic activity. Under photoirradiation, the surface of TiO2 particles shows hydrophilic property. This property prevents organic compounds from adsorbing on the surface of TiO2 photocatalysts in an aqueous media. This condition is a great disadvantage to a degradation of organic compounds in an aqueous media on the photoirradiation of TiO2 photocatalysts. In order to overcome the disadvantage, the surface of TiO2 particles was modified with hydrocarbon chains through Ti-O-Si bond. By introducing the hydrocarbon chains on the surface of TiO2 particles, the property of TiO2 surface becomes hydrophobic. We have been investigating to prepare TiO2 photocatalysts on which was modified with hydrocarbon chain, metal complex, and mocromolecules having molecular recognition sites.
Hybrid Photocatalyst No1
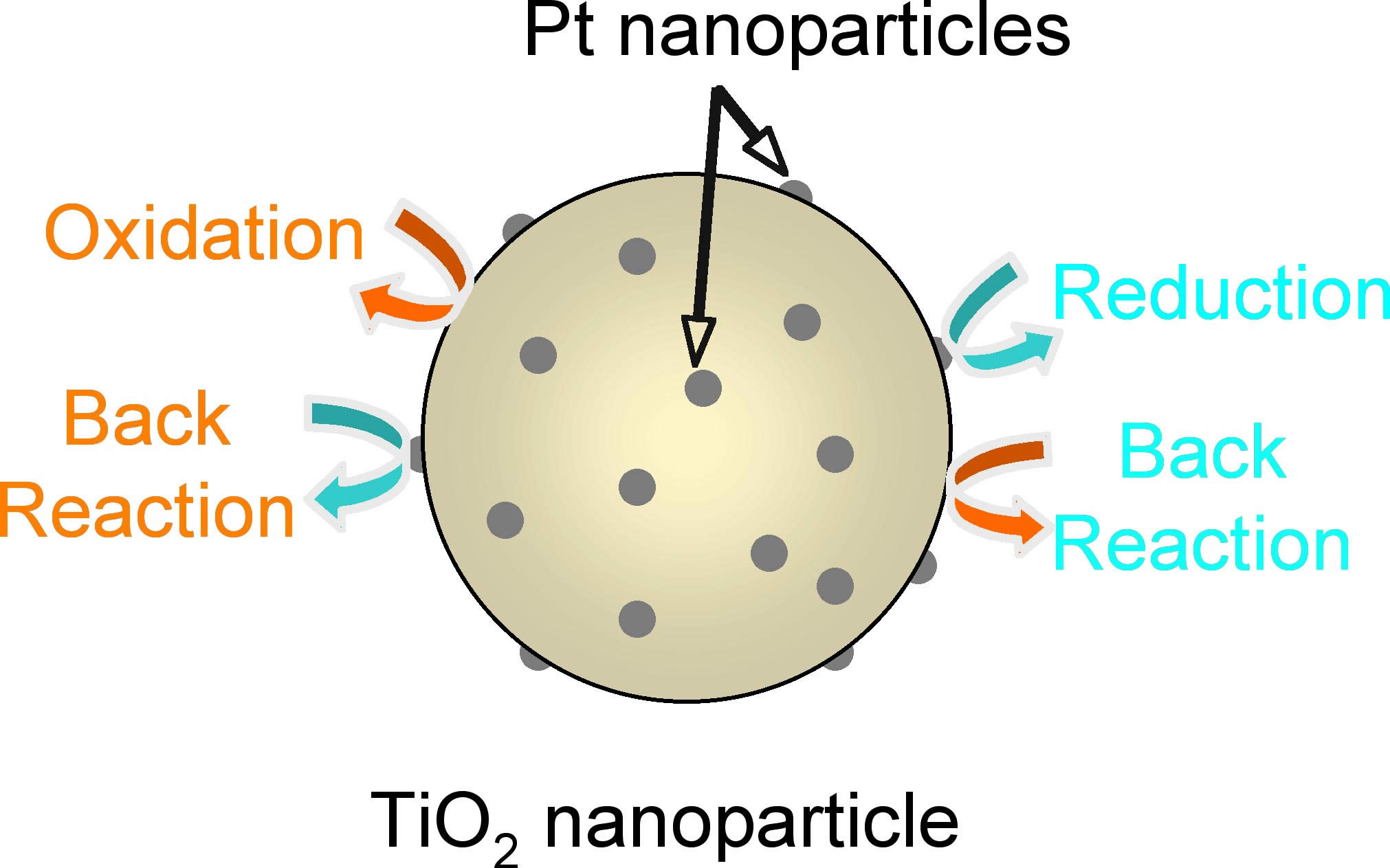
The most serious problems of photocatalysts such as TiO2 is back reaction. Both oxidation and reduction proceeded at the surface of the same particles. Under the condition, back reactions preceeded efficientlly resulting in siginificantly decreasing photocatalytic activity.
通常の酸化チタンナノ粒子の反応模式図
We developed site selective platinization of TiO2 nanotube. Pt nanoparticles
were deposited only inside TiO2 nanotube. Under the condition, reduction
mainly proceed inside TiO2 nanotube. On the ther hand, oxidation mainly
take place out side wall of nanotube. The photocatalytic activity of
Pt deposited TiO2 nanotube is much higher than than of usual Pt deposited
spherical TiO2 nanoparticles.
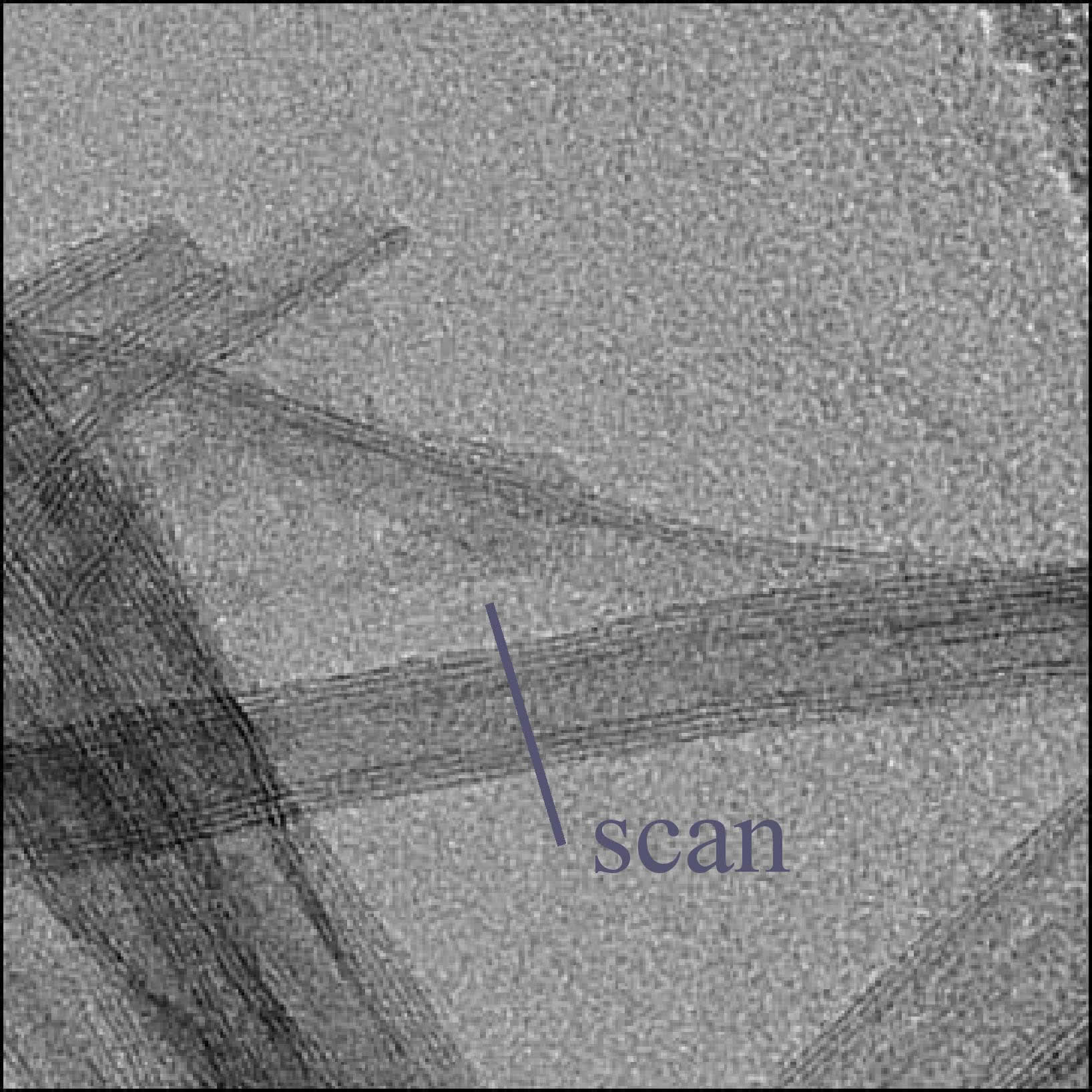
TEM image of TiO2 nanotube
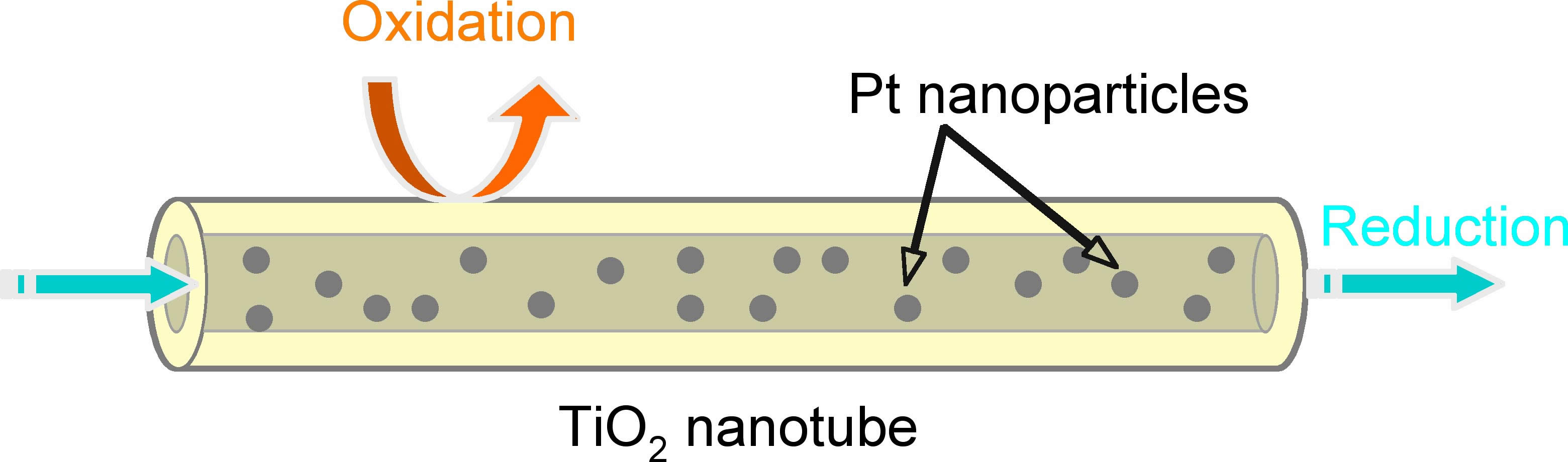
Schematic illustration of site selective Pt deposition
of TiO2 nanotube
Selective Oxidation of Organic Compounds using TiO2 photocatalysts and Molecular Oxygen
Photocatalytic oxidation of naphthalene, olephine, and adamantane etc. was investigated in a mixed solution of acetonitrile and water using various kinds of TiO2 powders as the photocatalysts and molecular oxygen as the electron acceptor. The main product from naphthalene is 2-formylcinnamaldehyde. For this reaction, anatase small TiO2 particles, which are commonly used as photocatalyst, are inactive, probably because band bending is necessary for the oxidation of naphthalene. If the particles are not extremely small, pure rutile and pure anatase powders show fairly high activity, and those containing both anatase and rutile phases show the highest activity. When a pure anatase powder is partly (about 90%) converted to the rutile form by heat treatment, the activity is largely enhanced. The activity of pure rutile particles is also enhanced by physically mixing them with a small amount of small-sized anatase particles, which are inactive for this reaction. These results can be explained by the synergism between rutile and anatase particles. We consider that electrons are transferred from rutile particles to anatase particles, i.e., naphthalene is mainly oxidized on rutile particles and oxygen is mainly reduced on anatase particles. This electron transfer process is supported by electrochemical properties of TiO2 electrodes for reduction of oxygen.